No products in the cart.
Registration Opens for the New Biosimilar & Biologic Specialist (CBBS) Examination
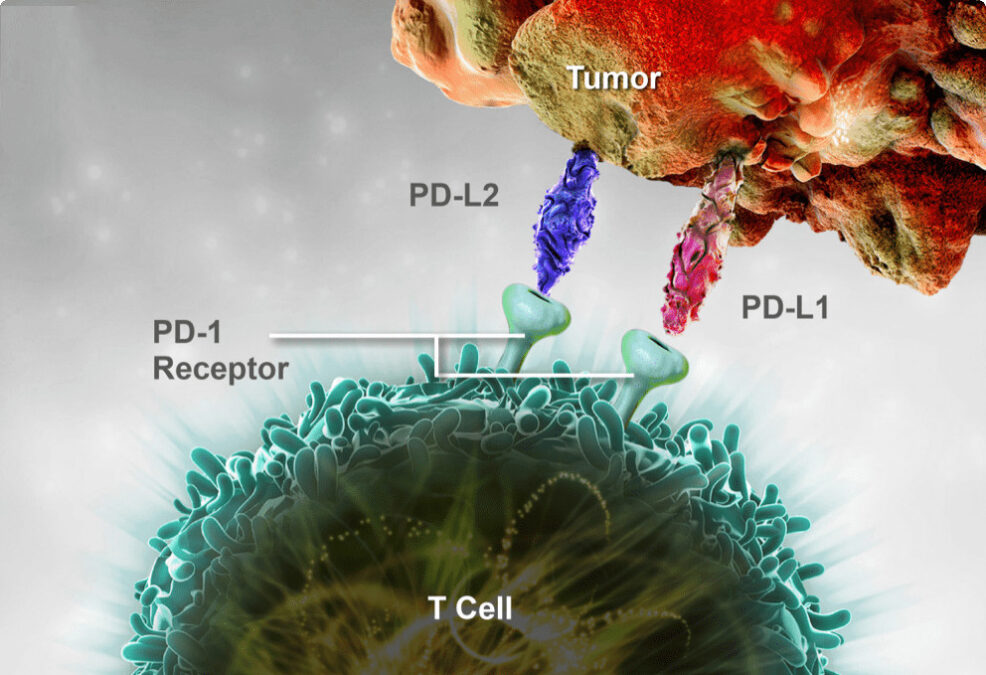
The past two decades have witnessed a quiet revolution in medicine. The advent of biologic therapies has transformed the treatment of complex, debilitating conditions, offering new hope to millions of patients with cancer, autoimmune diseases, and rare genetic disorders. These powerful, large-molecule drugs represent the pinnacle of modern pharmaceutical science. Today, this revolution is entering a new and profoundly complex phase: the era of the biosimilar.
As blockbuster biologics reach their patent cliffs, a wave of highly similar, cost-effective biosimilars is entering the market, creating one of the most significant clinical and financial opportunities in modern healthcare. The pharmacist is the central figure in navigating this new landscape, tasked with making critical, multi-million-dollar decisions that impact patient access, institutional budgets, and clinical outcomes. This role requires a sophisticated, hybrid expertise in clinical science, regulatory affairs, and pharmacoeconomics.
To validate this highly specialized and increasingly vital expertise, the Council on Pharmacy Standards (CPS) is proud to announce that registration is now officially open for the inaugural examination for the Certified Biosimilar & Biologic Specialist (CBBS).
The New Era of Biologics and the Need for a New Standard
The rise of biosimilars is fundamentally reshaping the pharmacy landscape. Unlike the straightforward world of small-molecule generics, the science and regulation of biosimilars are far more nuanced. Decisions about formulary placement, therapeutic interchangeability, and patient and provider education are complex and carry significant weight.
Health systems and managed care organizations are facing critical questions:
How do we critically evaluate the clinical data to ensure a biosimilar is a safe and effective alternative for our patients?
What is the operational strategy for a successful, system-wide transition from a reference product to a preferred biosimilar?
How do we educate physicians and patients to build trust and ensure confidence in these new therapies?
How do we navigate payer policies and GPO contracts to maximize the financial benefits of a biosimilar strategy?
Answering these questions effectively requires a level of expertise that goes far beyond standard clinical training. It demands a deep understanding of protein chemistry, FDA regulatory pathways, immunogenicity, and health economics. The Certified Biosimilar & Biologic Specialist (CBBS) certification has been meticulously developed to be the definitive national standard for this essential expertise. It is designed to identify the professionals who can lead their organizations with confidence through this new and complex therapeutic frontier.
What is a Certified Biosimilar & Biologic Specialist? A Deep Dive into the CBBS Domains
The CBBS examination is built upon a comprehensive Job Task Analysis conducted with national leaders in managed care, health-system pharmacy, and the biopharmaceutical industry. The credential validates a candidate’s mastery across four critical domains.
Domain 1: The Scientific and Regulatory Landscape
A true expert must understand the fundamental science that makes biologics and biosimilars unique. This domain covers the core principles of protein manufacturing, characterization, and the inherent structural complexities of large-molecule drugs. Crucially, it assesses a deep, working knowledge of the specific FDA regulatory pathway for biosimilars (the 351(k) pathway), including a clear understanding of concepts like analytical similarity, extrapolation of indications, and the rigorous data requirements for achieving an “interchangeable” designation.
Domain 2: Clinical and Therapeutic Assessment
This domain focuses on the practical application of clinical evidence. A CBBS-certified professional can critically evaluate the totality of the evidence for a biosimilar, including the analytical, nonclinical, and clinical data. They understand the science of immunogenicity, can assess its potential clinical impact, and are able to articulate the risks and benefits of switching a stable patient from a reference product to a biosimilar. This includes developing clinical guidelines and monitoring plans for patients undergoing a transition.
Domain 3: Formulary Management and Pharmacoeconomics
This is the critical business-focused domain. A certified specialist can conduct sophisticated pharmacoeconomic analyses to model the budget impact of a biosimilar adoption strategy. They understand the complex interplay between manufacturer rebates, GPO contracts, and payer coverage policies. Competencies in this area include developing institutional formulary policies, navigating the challenges of “non-medical switching,” and effectively negotiating with payers and pharmaceutical manufacturers.
Domain 4: Patient, Provider, and System Implementation
Knowledge without effective implementation is powerless. This domain validates the skills required to lead a successful biosimilar strategy from concept to execution. This includes developing effective educational materials for physicians, pharmacists, and patients to build confidence in biosimilars. It also covers the operational aspects of implementation, such as updating electronic health record order sets, managing inventory transitions, and tracking the clinical and financial outcomes of a biosimilar initiative.
Who Should Pursue the CBBS Certification?
This specialized certification is designed for pharmacy professionals who are directly involved in making high-level clinical and financial decisions about biologic and biosimilar therapies. Ideal candidates include:
Formulary Management Pharmacists at health systems or payers
Managed Care Pharmacists involved in clinical program development
Specialty Pharmacists in health-system, PBM, or independent settings
Clinical Pharmacists practicing in rheumatology, gastroenterology, dermatology, and oncology
Pharmacy Leaders (Directors, Managers) responsible for the pharmacy budget
Pharmacists in the Pharmaceutical/Biotech Industry (e.g., Medical Affairs, HEOR)
A Word from a National Expert
“The decisions we are making around biologics and biosimilars are some of the highest-impact decisions in modern pharmacy, affecting both our annual budget by tens of millions of dollars and the lives of our most complex patients,” notes a Vice President of Pharmacy Services at a large integrated delivery network who served as the CBBS SME Chair. “Having a national certification like the CBBS provides a clear, defensible benchmark for the advanced expertise we need on our teams. It’s an essential credential for the future of pharmacy leadership and strategic management.”
Your Opportunity to Be a Pioneer: Inaugural Exam Details
The launch of the CBBS certification marks a pivotal moment for the pharmacy profession. We invite you to be among the first to earn this prestigious credential and establish yourself as a recognized national leader in this critical and rapidly growing field.
Eligibility: Candidates must meet the current CPS eligibility requirements, including at least one year of relevant professional experience or completion of a recognized formal training program (e.g., PGY residency). Experience in managed care, formulary management, or specialty pharmacy is highly relevant.
Candidate Handbook: The official CBBS Candidate Handbook, which includes a detailed examination content outline and sample questions, is now available for download on our website.
Registration Window: Registration for the inaugural examination is now open and will close on January 15, 2025.
Inaugural Testing Window: The first-ever CBBS examination will be administered from March 1, 2025, to April 15, 2025.
The era of biologics and biosimilars is here. The need for true, validated expertise has never been greater. Be among the first to prove your knowledge and lead your organization with confidence into this new frontier.
Register for the inaugural CBBS examination today.

